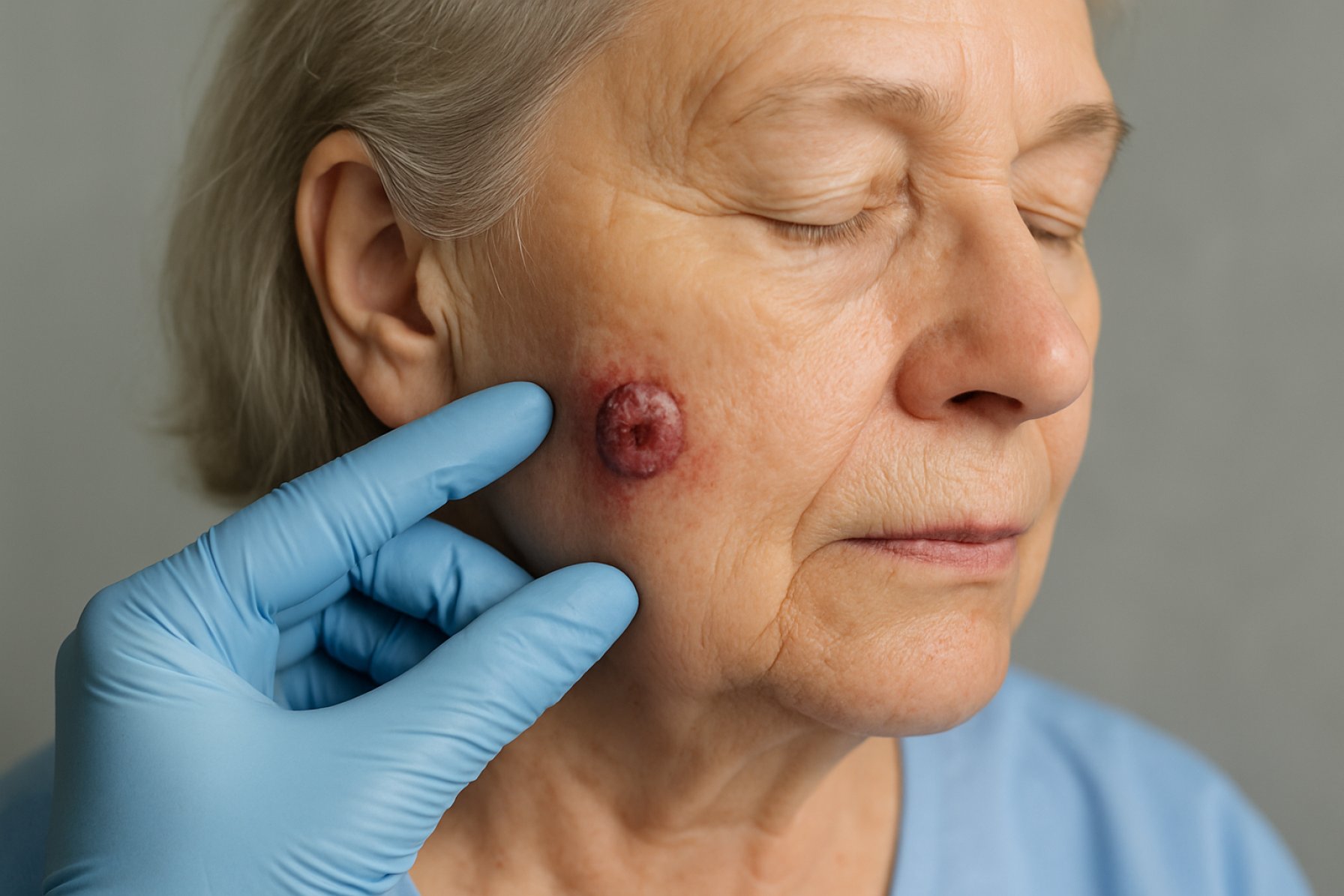
Merkel Cell Carcinoma Immunotherapy Research in 2025: Unveiling Next-Gen Therapies and Market Dynamics. Explore the Innovations and Strategic Shifts Shaping the Future of Rare Skin Cancer Treatment.
- Executive Summary: Key Trends and Market Drivers
- Epidemiology and Unmet Needs in Merkel Cell Carcinoma
- Current Immunotherapy Landscape: Approved Treatments and Leading Candidates
- Pipeline Analysis: Emerging Therapies and Clinical Trial Highlights
- Key Players and Strategic Collaborations (e.g., merck.com, bms.com, pfizer.com)
- Regulatory Milestones and Policy Developments
- Market Forecast 2025–2030: Growth Projections and Regional Insights
- Technological Innovations: Biomarkers, Delivery Systems, and Combination Approaches
- Challenges and Barriers: Resistance, Safety, and Access
- Future Outlook: Investment Opportunities and Strategic Recommendations
- Sources & References
Executive Summary: Key Trends and Market Drivers
Merkel cell carcinoma (MCC) is a rare but aggressive skin cancer, and immunotherapy has rapidly emerged as the cornerstone of advanced MCC treatment. As of 2025, the field is characterized by robust clinical research, regulatory momentum, and expanding access to novel immunotherapeutic agents. The approval and clinical integration of immune checkpoint inhibitors, particularly PD-1 and PD-L1 inhibitors, have transformed the therapeutic landscape, driving significant improvements in patient outcomes and fueling further innovation.
Avelumab, a PD-L1 inhibitor, remains the only agent with full regulatory approval for metastatic MCC in multiple major markets, following its landmark approval by the Merck KGaA and Pfizer Inc. partnership. Pembrolizumab, a PD-1 inhibitor developed by Merck & Co., Inc. (known as MSD outside the US and Canada), is also widely used off-label and in clinical trials, with ongoing studies aiming to expand its indications and optimize treatment regimens. These agents have demonstrated durable responses and improved survival rates compared to historical chemotherapy, establishing immunotherapy as the new standard of care for advanced MCC.
Key trends shaping the MCC immunotherapy research landscape in 2025 include:
- Expansion of clinical trials: Numerous phase II and III studies are underway, evaluating novel checkpoint inhibitors, combination regimens (e.g., with radiation or targeted therapies), and next-generation immunomodulators. Leading pharmaceutical companies such as Bristol Myers Squibb and F. Hoffmann-La Roche AG are actively investigating new agents and combinations to overcome resistance and improve efficacy.
- Biomarker-driven research: Efforts to identify predictive biomarkers, such as tumor mutational burden and viral status (Merkel cell polyomavirus), are intensifying, aiming to personalize immunotherapy and maximize patient benefit.
- Regulatory and reimbursement advances: Accelerated approvals and expanded reimbursement for immunotherapies in MCC are being observed in North America, Europe, and parts of Asia-Pacific, driven by compelling clinical data and advocacy from organizations like the National Cancer Institute.
- Global collaboration: International consortia and academic-industry partnerships are facilitating large-scale studies and real-world data collection, supporting evidence-based guidelines and broader patient access.
Looking ahead, the MCC immunotherapy market is expected to grow steadily through the late 2020s, propelled by ongoing research, new drug launches, and increasing awareness among clinicians. The focus will likely shift toward optimizing sequencing, combination strategies, and expanding indications into earlier disease stages, with the ultimate goal of improving long-term survival and quality of life for MCC patients.
Epidemiology and Unmet Needs in Merkel Cell Carcinoma
Merkel cell carcinoma (MCC) is a rare but highly aggressive neuroendocrine skin cancer, with an incidence that has been rising globally over the past decade. In 2025, the annual incidence in the United States is estimated at approximately 0.7 cases per 100,000 persons, translating to over 2,500 new cases each year. The disease predominantly affects older adults and immunosuppressed individuals, with a higher prevalence in Caucasian populations. Despite advances in early detection, MCC is often diagnosed at advanced stages due to its rapid growth and nonspecific presentation.
The prognosis for advanced MCC remains poor, with five-year survival rates below 20% for metastatic disease. Traditional treatments, such as surgery and radiotherapy, are effective in localized cases but offer limited benefit in advanced or recurrent settings. Chemotherapy, once the mainstay for metastatic MCC, is associated with high relapse rates and significant toxicity, underscoring the urgent need for more effective and durable therapies.
Immunotherapy has emerged as a transformative approach in MCC management, particularly with the advent of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis. Agents such as avelumab and pembrolizumab have demonstrated durable responses and improved survival in both first-line and previously treated patients. However, a substantial proportion of patients—estimated at 40-50%—either do not respond or eventually develop resistance to these therapies. This highlights a significant unmet need for novel immunotherapeutic strategies and combination regimens.
Current research in 2025 is focused on several key areas to address these gaps. Investigational approaches include next-generation checkpoint inhibitors, bispecific antibodies, and adoptive cell therapies such as tumor-infiltrating lymphocytes (TILs) and CAR-T cells. Additionally, there is growing interest in therapeutic vaccines targeting Merkel cell polyomavirus (MCPyV), which is implicated in the majority of MCC cases. Biomarker-driven patient selection and the identification of resistance mechanisms are also active areas of investigation, aiming to personalize immunotherapy and improve outcomes.
Despite the progress, access to immunotherapy remains uneven, particularly outside major academic centers and in low-resource settings. Ongoing efforts by industry leaders such as Merck KGaA (developer of avelumab) and Merck & Co., Inc. (developer of pembrolizumab) are focused on expanding clinical trial networks and accelerating regulatory approvals worldwide. Collaborative initiatives with organizations like the National Cancer Institute are also underway to improve epidemiological surveillance and facilitate access to cutting-edge therapies.
Looking ahead, the next few years are expected to bring further advances in MCC immunotherapy, with ongoing clinical trials poised to define new standards of care. However, the rarity and heterogeneity of MCC continue to pose challenges for research and drug development, reinforcing the need for international collaboration and innovative trial designs.
Current Immunotherapy Landscape: Approved Treatments and Leading Candidates
Merkel cell carcinoma (MCC) is a rare but aggressive neuroendocrine skin cancer with a high propensity for recurrence and metastasis. Over the past decade, immunotherapy has transformed the treatment landscape for advanced MCC, and as of 2025, research and clinical practice continue to evolve rapidly.
The first major breakthrough in MCC immunotherapy was the approval of avelumab, a PD-L1 inhibitor, by the U.S. Food and Drug Administration (FDA) in 2017 for metastatic MCC. Avelumab, developed by Merck KGaA (in partnership with Pfizer), remains a cornerstone of treatment for advanced cases. Clinical trials have demonstrated durable responses, with some patients achieving long-term remission. Pembrolizumab, a PD-1 inhibitor from Merck & Co., Inc. (known as MSD outside the U.S. and Canada), is also approved for recurrent locally advanced or metastatic MCC, further expanding immunotherapeutic options.
As of 2025, these checkpoint inhibitors are standard of care for advanced MCC, and ongoing studies are refining their use in earlier disease stages and in combination regimens. Notably, both avelumab and pembrolizumab are being evaluated in adjuvant and neoadjuvant settings, with early data suggesting improved recurrence-free survival when used before or after surgery in high-risk patients.
Beyond approved agents, several promising candidates are in late-stage development. Nivolumab, another PD-1 inhibitor from Bristol Myers Squibb, is under investigation for MCC, with phase II/III trials assessing its efficacy as monotherapy and in combination with other immunomodulators. Additionally, novel approaches such as bispecific antibodies, T-cell engagers, and personalized cancer vaccines are entering early-phase clinical trials, aiming to overcome resistance mechanisms and broaden the patient population benefiting from immunotherapy.
The field is also witnessing increased collaboration between academic centers and industry to identify biomarkers predictive of response, such as tumor mutational burden and viral status (Merkel cell polyomavirus). These efforts are expected to inform patient selection and optimize therapeutic strategies in the coming years.
Looking ahead, the immunotherapy landscape for MCC is poised for further expansion. Combination regimens, next-generation checkpoint inhibitors, and cellular therapies are anticipated to enter pivotal trials by 2026–2027. The continued commitment of leading biopharmaceutical companies, including Merck KGaA, Merck & Co., Inc., and Bristol Myers Squibb, ensures robust innovation and the potential for improved outcomes in this challenging malignancy.
Pipeline Analysis: Emerging Therapies and Clinical Trial Highlights
The landscape of immunotherapy research for Merkel cell carcinoma (MCC) continues to evolve rapidly as of 2025, with a robust pipeline of emerging therapies and a growing number of clinical trials targeting this aggressive skin cancer. The focus remains on harnessing immune checkpoint inhibitors, novel monoclonal antibodies, and combination regimens to improve outcomes for patients with advanced or refractory MCC.
Checkpoint inhibitors, particularly those targeting the PD-1/PD-L1 axis, have established themselves as the backbone of MCC immunotherapy. Merck & Co., Inc. (known as MSD outside the US and Canada) leads the field with pembrolizumab, a PD-1 inhibitor that received FDA approval for metastatic MCC and continues to be evaluated in multiple ongoing trials for earlier-stage disease and combination strategies. Similarly, Pfizer Inc. and Merck KGaA (Germany) jointly market avelumab, a PD-L1 inhibitor, which remains the only agent with full approval for metastatic MCC in several regions. Both agents are being studied in adjuvant and neoadjuvant settings, with interim data suggesting improved recurrence-free survival and durable responses.
Beyond established checkpoint inhibitors, the pipeline features next-generation immunotherapies. Bristol Myers Squibb is investigating nivolumab, another PD-1 inhibitor, in combination with other immune modulators. Early-phase trials are also exploring novel targets such as LAG-3 and TIGIT, with companies like Novartis and Roche evaluating bispecific antibodies and immune agonists in MCC cohorts. These approaches aim to overcome resistance mechanisms and broaden the pool of patients who benefit from immunotherapy.
Adoptive cell therapies and therapeutic vaccines are emerging as promising modalities. Several academic centers and biotechnology firms are advancing T-cell receptor (TCR) and chimeric antigen receptor (CAR) T-cell therapies tailored to MCC antigens, though these remain in early clinical development. Additionally, oncolytic virus therapies, such as those under investigation by Amgen Inc., are being tested in combination with checkpoint blockade to enhance tumor immunogenicity.
Looking ahead, the next few years are expected to yield pivotal data from randomized trials evaluating combination regimens, novel immune targets, and the integration of immunotherapy into earlier lines of MCC management. The field is also moving toward biomarker-driven approaches to better select patients and optimize therapeutic outcomes. As the pipeline matures, collaboration between industry leaders, academic institutions, and regulatory agencies will be critical to translating these advances into improved survival and quality of life for patients with Merkel cell carcinoma.
Key Players and Strategic Collaborations (e.g., merck.com, bms.com, pfizer.com)
The landscape of Merkel cell carcinoma (MCC) immunotherapy research in 2025 is shaped by a select group of pharmaceutical leaders and strategic collaborations, with a focus on advancing immune checkpoint inhibitors and novel immunomodulatory approaches. The most prominent players include Merck & Co., Inc. (known as MSD outside the United States and Canada), Bristol Myers Squibb, and Pfizer Inc., each leveraging their immuno-oncology portfolios and global research networks.
- Merck & Co., Inc.: Merck’s anti-PD-1 therapy, pembrolizumab (Keytruda), remains a cornerstone in MCC treatment, having received regulatory approvals for advanced MCC in multiple regions. Ongoing research in 2025 focuses on expanding indications, optimizing combination regimens, and investigating neoadjuvant and adjuvant settings. Merck is also engaged in collaborations with academic centers and biotech firms to explore synergistic effects with other immunotherapies and targeted agents.
- Bristol Myers Squibb: Bristol Myers Squibb’s nivolumab (Opdivo) and ipilimumab (Yervoy) are being evaluated in MCC, both as monotherapies and in combination. The company is actively involved in multicenter trials and has established partnerships with cooperative oncology groups to accelerate clinical development. BMS’s strategic alliances aim to address resistance mechanisms and improve durable response rates in MCC patients.
- Pfizer Inc.: Pfizer, through its alliance with Merck KGaA, Darmstadt, Germany, continues to develop avelumab (Bavencio), the first anti-PD-L1 antibody approved for metastatic MCC. In 2025, Pfizer is investing in real-world evidence studies and post-marketing surveillance to refine patient selection and long-term safety profiles. The company is also exploring next-generation immunotherapies and potential biomarkers for response prediction.
- Strategic Collaborations: The MCC immunotherapy field is characterized by cross-industry and academia-industry collaborations. Notably, partnerships between pharmaceutical companies and leading cancer research institutions facilitate access to patient cohorts, translational research, and biomarker discovery. These collaborations are expected to intensify through 2025 and beyond, with a focus on personalized immunotherapy and overcoming resistance.
Looking ahead, the competitive landscape is likely to see further consolidation and new entrants, particularly as smaller biotech firms with innovative immunomodulatory platforms seek partnerships or acquisition by established players. The ongoing commitment of Merck & Co., Inc., Bristol Myers Squibb, and Pfizer Inc. to MCC immunotherapy research, combined with strategic collaborations, is expected to drive significant advances in treatment options and patient outcomes over the next several years.
Regulatory Milestones and Policy Developments
The regulatory landscape for Merkel cell carcinoma (MCC) immunotherapy has evolved rapidly, with significant milestones achieved in recent years and several key developments anticipated through 2025 and beyond. MCC, a rare but aggressive skin cancer, has seen transformative progress due to the advent of immune checkpoint inhibitors, particularly those targeting the PD-1/PD-L1 pathway.
A landmark regulatory event was the accelerated approval of avelumab, a PD-L1 inhibitor, by the U.S. Food and Drug Administration (FDA) in 2017 for metastatic MCC. This was followed by the approval of pembrolizumab, a PD-1 inhibitor, in 2018. Both approvals were based on robust clinical trial data demonstrating durable responses in patients with advanced disease. These agents are manufactured by Merck KGaA (avelumab, in partnership with Pfizer) and Merck & Co., Inc. (pembrolizumab), respectively. The European Medicines Agency (EMA) and other regulatory bodies have since granted similar approvals, establishing immunotherapy as the standard of care for advanced MCC.
As of 2025, regulatory agencies are focusing on expanding indications for these therapies, including their use in earlier-stage disease and in combination regimens. Ongoing and recently completed trials are evaluating the efficacy of immunotherapies as adjuvant or neoadjuvant treatments, with regulatory submissions expected in the near future. The FDA and EMA have both signaled a willingness to consider expedited review pathways for these indications, given the high unmet need and promising early data.
Policy developments are also shaping the MCC immunotherapy landscape. The U.S. Centers for Medicare & Medicaid Services (CMS) has updated reimbursement policies to reflect the inclusion of immunotherapies in treatment guidelines, improving patient access. In Europe, the EMA is working with national health authorities to harmonize access and reimbursement for these high-cost therapies, aiming to reduce disparities across member states.
Looking ahead, regulatory agencies are expected to place greater emphasis on real-world evidence and post-marketing surveillance to monitor long-term safety and effectiveness. There is also growing interest in biomarker-driven regulatory strategies, with agencies encouraging the development of companion diagnostics to better identify patients most likely to benefit from immunotherapy.
In summary, the period through 2025 is marked by ongoing regulatory momentum, with agencies such as the FDA, EMA, and CMS playing pivotal roles in shaping access, safety monitoring, and the future direction of MCC immunotherapy research and clinical practice.
Market Forecast 2025–2030: Growth Projections and Regional Insights
The global market for Merkel cell carcinoma (MCC) immunotherapy is poised for significant growth between 2025 and 2030, driven by increasing incidence rates, advances in immuno-oncology, and expanding regulatory approvals. MCC, a rare but aggressive skin cancer, has seen a paradigm shift in treatment with the advent of immune checkpoint inhibitors, particularly programmed death-ligand 1 (PD-L1) and programmed death-1 (PD-1) inhibitors. The market outlook for the next five years is shaped by ongoing clinical research, new product launches, and regional healthcare infrastructure developments.
North America is expected to maintain its dominance in the MCC immunotherapy market through 2030, owing to high awareness, robust clinical trial activity, and early adoption of novel therapies. The United States, in particular, benefits from the presence of leading biopharmaceutical companies and a favorable regulatory environment. Merck & Co., Inc. (known as MSD outside the US and Canada) continues to lead with its anti-PD-1 therapy, pembrolizumab (Keytruda), which has received FDA approval for MCC and is being evaluated in additional studies for expanded indications and combination regimens. Similarly, Pfizer Inc. and its partner Merck KGaA (Germany) market avelumab (Bavencio), the first immunotherapy approved for metastatic MCC, and are investing in further research to enhance efficacy and safety profiles.
Europe is projected to witness steady growth, supported by increasing access to immunotherapies and collaborative research initiatives. The European Medicines Agency (EMA) has approved both pembrolizumab and avelumab for MCC, and ongoing post-marketing studies are expected to generate real-world evidence to support broader adoption. Germany, France, and the UK are leading contributors to clinical research and patient enrollment in MCC immunotherapy trials.
The Asia-Pacific region is anticipated to experience the fastest growth rate, albeit from a smaller base, as awareness of MCC rises and healthcare systems invest in advanced oncology treatments. Japan and Australia are at the forefront, with regulatory approvals and local clinical trials underway. Expansion into China and other emerging markets is expected as companies like Merck & Co., Inc. and Pfizer Inc. pursue broader global access strategies.
Looking ahead, the MCC immunotherapy market is forecast to grow at a compound annual growth rate (CAGR) in the high single digits through 2030. Key growth drivers include label expansions, combination therapy development, and improved diagnostic capabilities. However, challenges such as high treatment costs, limited patient populations, and the need for long-term efficacy data may temper market acceleration. Strategic collaborations between pharmaceutical companies, academic centers, and healthcare providers will be crucial in shaping the market landscape and improving patient outcomes over the forecast period.
Technological Innovations: Biomarkers, Delivery Systems, and Combination Approaches
Merkel cell carcinoma (MCC) is a rare but aggressive skin cancer, and immunotherapy has emerged as a transformative approach in its management. As of 2025, technological innovations are rapidly advancing the field, particularly in the areas of biomarkers, delivery systems, and combination immunotherapy strategies.
Biomarker Development
The identification and validation of predictive biomarkers are central to optimizing immunotherapy for MCC. Recent research has focused on tumor mutational burden (TMB), PD-L1 expression, and the presence of Merkel cell polyomavirus (MCPyV) DNA as potential biomarkers for response to immune checkpoint inhibitors. Companies such as Roche and Agilent Technologies are developing advanced diagnostic assays to quantify these markers, enabling more personalized treatment regimens. Additionally, next-generation sequencing platforms are being refined to detect minimal residual disease and monitor immune response dynamics in real time.
Innovative Delivery Systems
Efficient delivery of immunotherapeutic agents remains a challenge, especially for patients with advanced or metastatic MCC. Nanoparticle-based delivery systems and liposomal formulations are under investigation to enhance the bioavailability and tumor targeting of immune modulators. Pfizer and Bristol Myers Squibb are among the pharmaceutical leaders exploring these technologies, aiming to reduce systemic toxicity and improve therapeutic outcomes. Furthermore, intratumoral injection techniques are being optimized to localize immune activation and minimize off-target effects.
Combination Immunotherapy Approaches
Combination strategies are at the forefront of MCC immunotherapy research. The integration of immune checkpoint inhibitors (such as anti-PD-1/PD-L1 antibodies) with other modalities—including oncolytic viruses, cancer vaccines, and targeted therapies—is being actively pursued. Merck & Co., Inc. (known for pembrolizumab) and Novartis are conducting clinical trials to evaluate the efficacy of these combinations in both first-line and refractory settings. Early-phase data suggest that such approaches may overcome resistance mechanisms and extend durable responses in a broader patient population.
Outlook for 2025 and Beyond
Looking ahead, the integration of artificial intelligence and machine learning into biomarker discovery and patient stratification is expected to accelerate progress. Collaborative efforts between academic centers, biotechnology firms, and pharmaceutical companies are likely to yield novel immunotherapeutic agents and companion diagnostics. As regulatory agencies continue to prioritize rare cancers, the pace of innovation in MCC immunotherapy is set to intensify, with the potential to significantly improve patient outcomes in the coming years.
Challenges and Barriers: Resistance, Safety, and Access
Merkel cell carcinoma (MCC) is a rare but aggressive skin cancer, and immunotherapy—particularly immune checkpoint inhibitors—has transformed its treatment landscape. However, as of 2025, several challenges and barriers persist in the research and clinical application of immunotherapy for MCC, notably in the areas of resistance, safety, and patient access.
Resistance to Immunotherapy remains a significant hurdle. While agents such as avelumab and pembrolizumab have demonstrated durable responses in a subset of patients, a considerable proportion either do not respond initially (primary resistance) or develop resistance after an initial response (acquired resistance). The mechanisms underlying resistance are multifactorial, involving tumor-intrinsic factors such as low tumor mutational burden, loss of antigen presentation machinery, and immunosuppressive tumor microenvironments. Ongoing research in 2025 is focused on identifying predictive biomarkers and developing combination strategies—such as pairing checkpoint inhibitors with oncolytic viruses, cytokines, or targeted therapies—to overcome resistance. Companies like Merck KGaA (developer of avelumab) and Merck & Co., Inc. (developer of pembrolizumab) are actively involved in these efforts, sponsoring clinical trials and translational research collaborations.
Safety Concerns are another barrier, as immune checkpoint inhibitors can trigger immune-related adverse events (irAEs) affecting organs such as the skin, liver, lungs, and endocrine glands. While most irAEs are manageable, severe cases can be life-threatening and require immunosuppressive therapy, which may compromise anti-tumor efficacy. The elderly and immunocompromised populations—who represent a significant proportion of MCC patients—are particularly vulnerable. In 2025, research is ongoing to refine patient selection, optimize dosing regimens, and develop early detection protocols for irAEs. Both Merck KGaA and Merck & Co., Inc. provide safety data and guidelines for clinicians, and are investing in post-marketing surveillance to better characterize long-term risks.
Access to Immunotherapy remains uneven globally. High costs, limited reimbursement, and regulatory hurdles restrict access in many regions. In the United States and Europe, avelumab and pembrolizumab are approved for advanced MCC, but in lower-resource settings, access is often limited by infrastructure and cost constraints. Efforts by manufacturers, such as patient assistance programs and expanded access initiatives, are ongoing but not universally available. Additionally, the rarity of MCC poses challenges for clinical trial enrollment and data generation, slowing the pace of innovation. Organizations such as U.S. Food and Drug Administration and European Medicines Agency continue to work with industry to streamline approval pathways and encourage research in rare cancers.
Looking ahead, overcoming these challenges will require coordinated efforts among pharmaceutical companies, regulatory agencies, and healthcare systems to ensure that advances in immunotherapy translate into improved outcomes for all MCC patients.
Future Outlook: Investment Opportunities and Strategic Recommendations
The landscape of Merkel cell carcinoma (MCC) immunotherapy research is poised for significant evolution in 2025 and the coming years, presenting a range of investment opportunities and strategic considerations for stakeholders. MCC, a rare but aggressive skin cancer, has seen transformative advances with the advent of immune checkpoint inhibitors, particularly anti-PD-1 and anti-PD-L1 therapies. The continued expansion of clinical trials, regulatory approvals, and biomarker-driven approaches is expected to shape the sector’s trajectory.
Key players such as Merck & Co., Inc. (known as MSD outside the US and Canada) and Pfizer Inc. have established a strong presence in the MCC immunotherapy market. Merck’s pembrolizumab (Keytruda) and Pfizer’s avelumab (Bavencio, co-developed with EMD Serono, the biopharmaceutical business of Merck KGaA, Darmstadt, Germany) are the only immune checkpoint inhibitors with regulatory approval for advanced MCC in multiple regions. Both companies are actively investing in expanding indications, combination regimens, and real-world evidence generation to reinforce their market positions.
Looking ahead, investment opportunities are likely to center on:
- Next-generation immunotherapies: Companies are exploring novel checkpoint inhibitors, bispecific antibodies, and cell-based therapies. Early-stage biotech firms and established players are expected to seek partnerships and licensing deals to accelerate pipeline development.
- Biomarker-driven strategies: The identification of predictive biomarkers for response and resistance to immunotherapy is a priority. Investment in companion diagnostics and precision medicine platforms will be critical for optimizing patient selection and improving outcomes.
- Geographic expansion: As regulatory approvals broaden in Asia-Pacific and Latin America, companies with robust global infrastructure, such as F. Hoffmann-La Roche Ltd and Bristol Myers Squibb, are well-positioned to capture emerging market share through strategic alliances and local partnerships.
- Combination regimens: Ongoing trials are evaluating the synergy of immunotherapies with radiation, chemotherapy, and targeted agents. Investors should monitor data readouts from these studies, as positive results could drive new standards of care and expand addressable patient populations.
Strategically, stakeholders should prioritize investments in companies with diversified immuno-oncology pipelines, strong intellectual property portfolios, and demonstrated expertise in regulatory navigation. Collaborations between pharmaceutical leaders, academic centers, and technology innovators will be essential to accelerate translational research and clinical adoption. As the competitive landscape intensifies, early engagement in licensing, co-development, and commercialization agreements will be key to securing long-term value in the MCC immunotherapy sector.
Sources & References
- Merck & Co., Inc.
- Bristol Myers Squibb
- F. Hoffmann-La Roche AG
- National Cancer Institute
- Merck & Co., Inc.
- Novartis
- European Medicines Agency